Meaning Of Robustness In Method Of Validation
In this part of the course the robustness and ruggedness are introduced and explained.

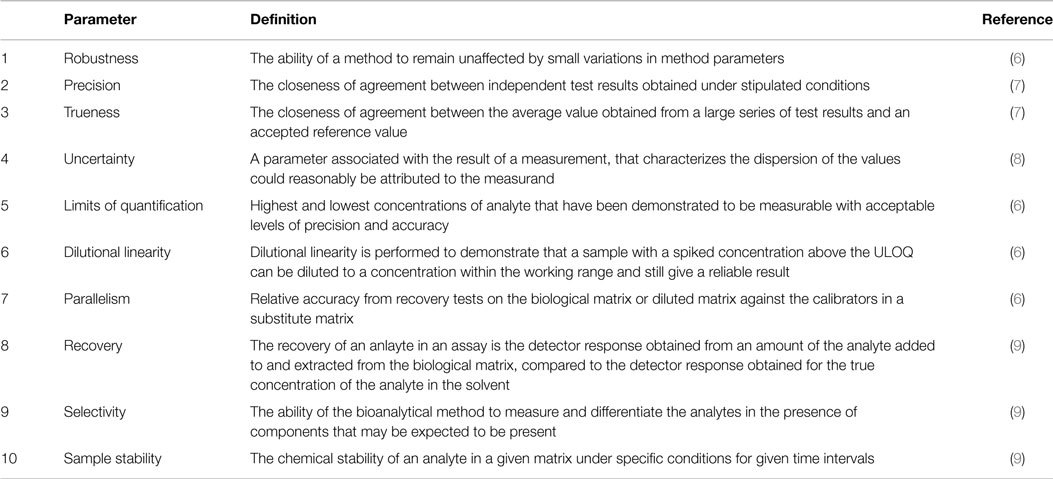
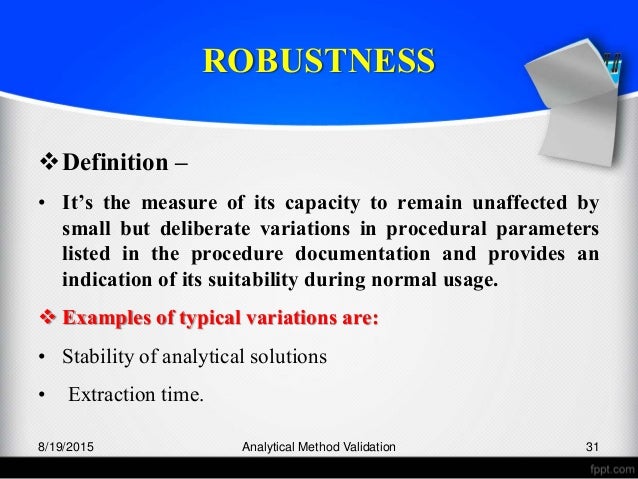
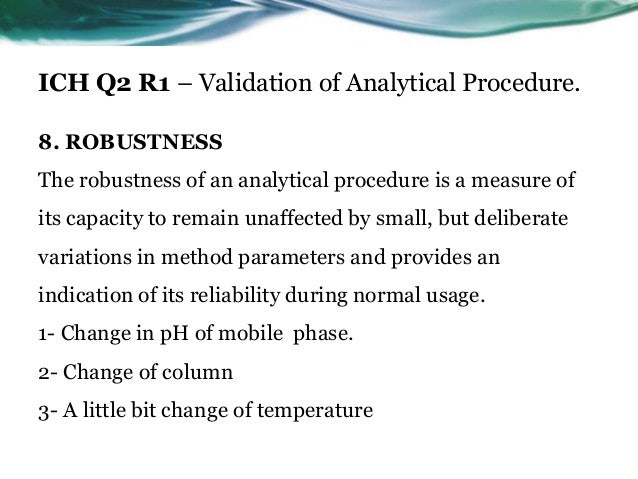
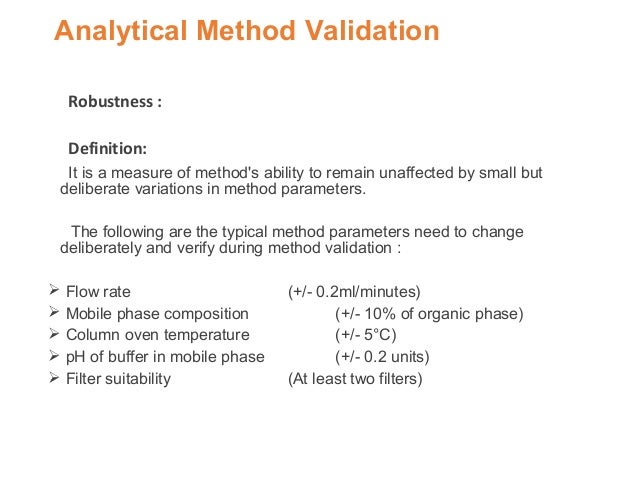
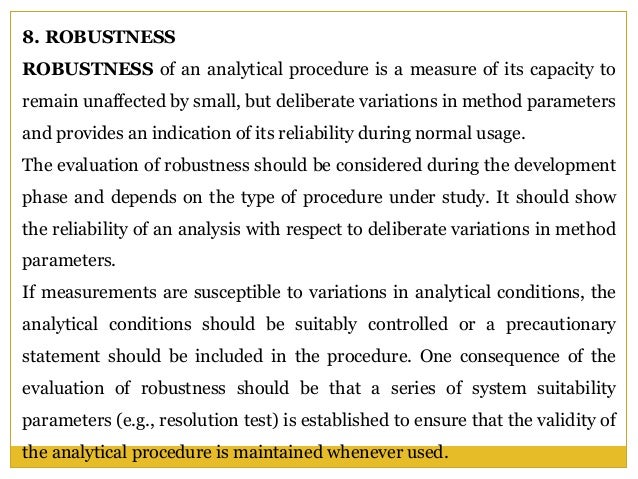
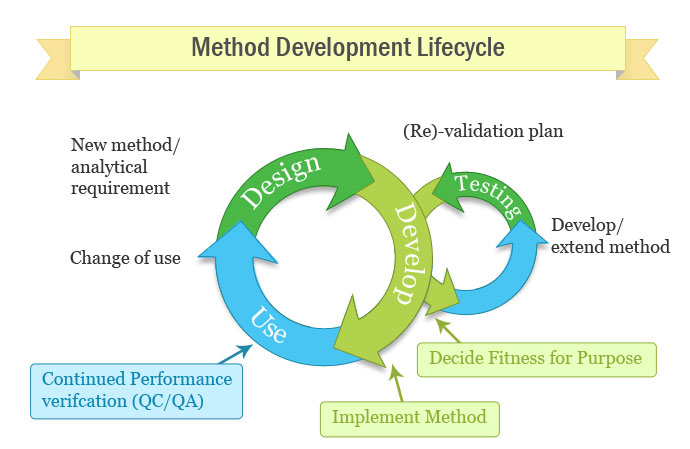
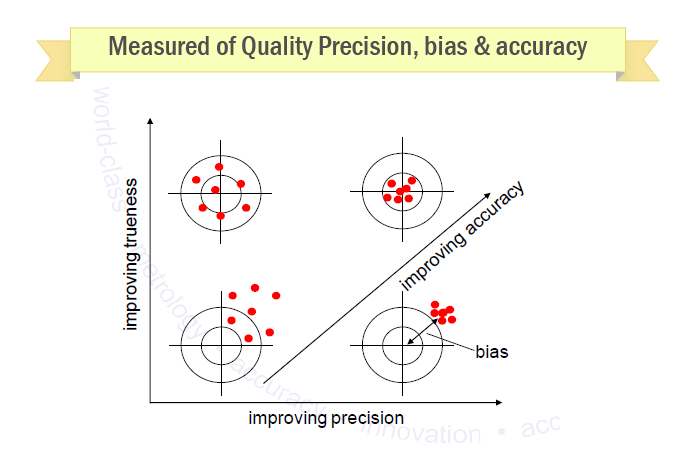
Meaning of robustness in method of validation. Robustness traditionally has not been considered as a validation parameter in the strictest sense because usually it is investigated during method development once the method is at least partially optimized. In computer science robustness is the ability of a computer system to cope with errors during execution and cope with erroneous input. Robustness is the evaluation of an analytical method wherein the results obtained are found to be reliable even when performed in a slightly varied condition. In the usp the robustness of an analytical procedure is defined as a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability in normal usage.
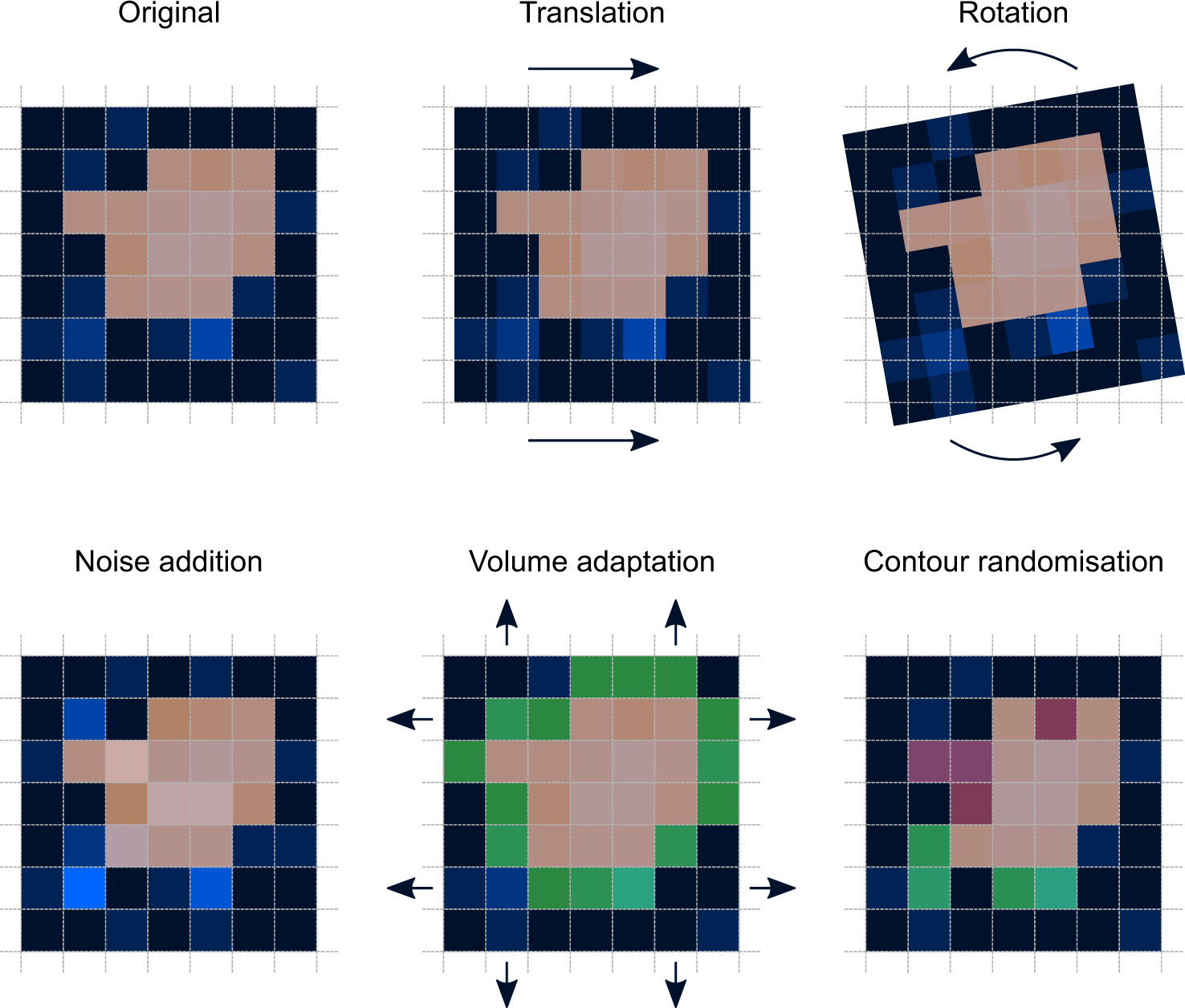
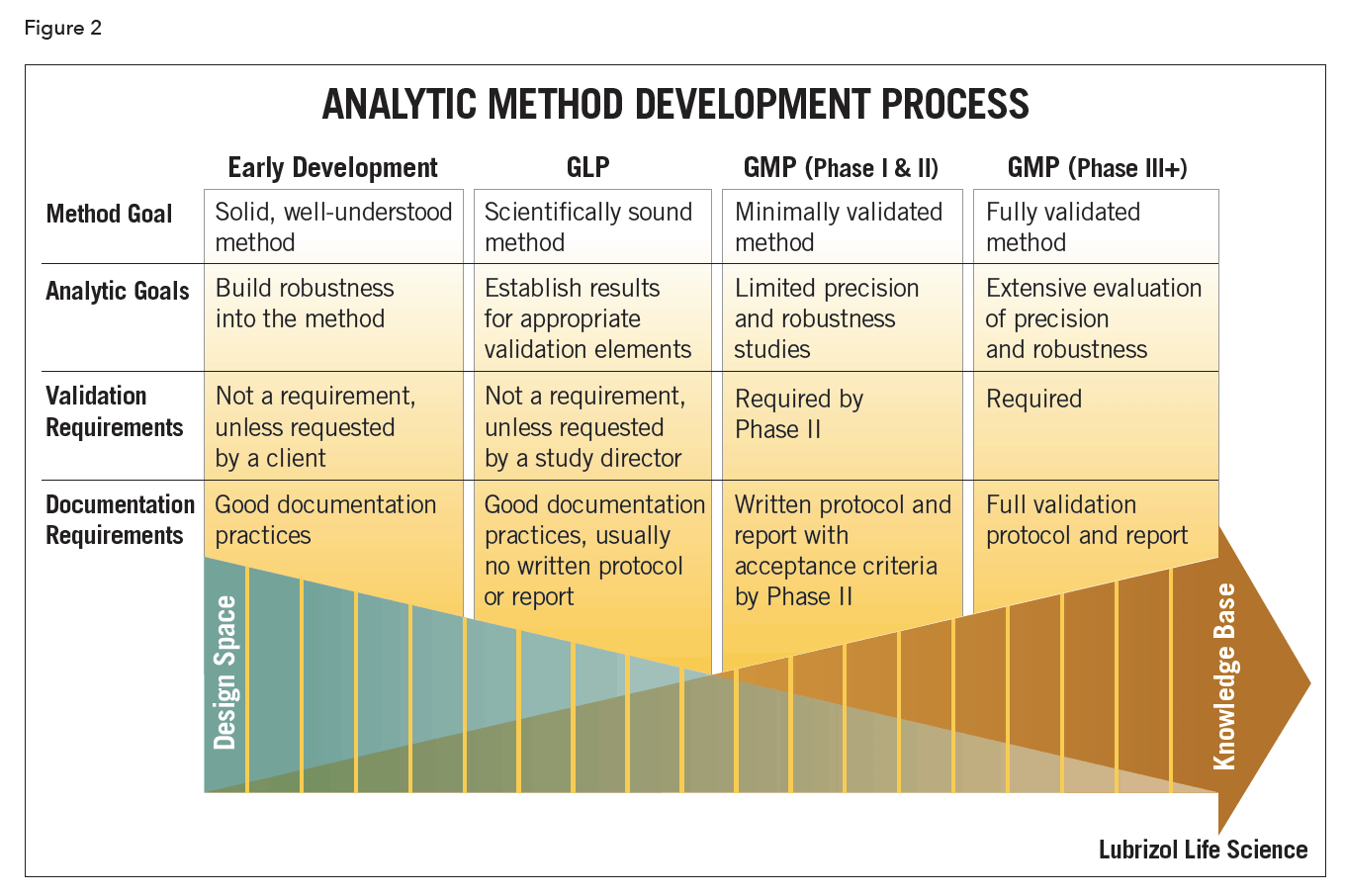
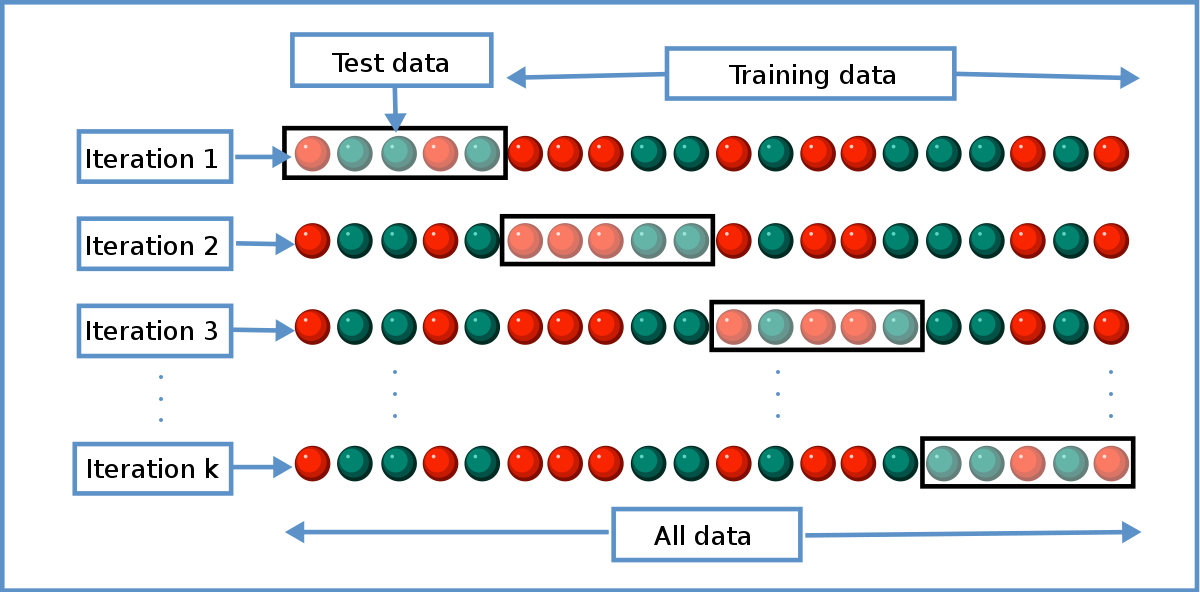
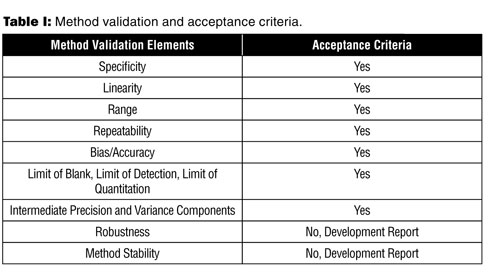
Robustness data obtained during a methods development can be submitted in support of the validation of a method. The purpose of a robustness study is to find out as much as possible about potential issues with a new analytical method and thus how it will perform in routine use. Usually we deliberately make changes in the method parameters to see if the method can still generate valid data. In this article we will address the same question through the parameter called robustness which can be evaluated during method validation if not yet done earlier eg.
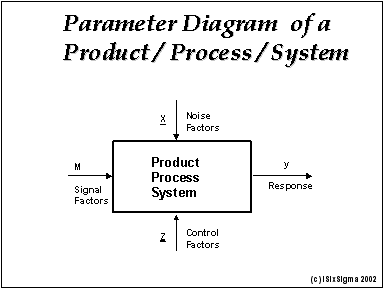
Robustness can encompass many areas of computer science such as robust programming robust machine learning and robust security networkformal techniques such as fuzz testing are essential to showing robustness since this type of testing involves invalid. The terms robustness and ruggedness refer to the ability of an analytical method to remain unaffected by small variations in method parameters mobile phase composition column age column temperature etc and influential environmental factors room temperature air humidity etc and characterize its reliability during normal usage. Ruggednessshould be used as a parameter evaluating constancy of the results when external factors such as analyst laboratory instrument reagents and days are varied robustnessshould be used as a parameter characterizing the stability of the method with respect to variations of the internal factors parameters of the method.